This paper has been published in which Prof. Terauchi is the corresponding author.
Natsume, S., Sugihara, Y., Kudoh, A., Oikawa, K., Shimizu, M., Ishikawa, Y., Nishihara, M., Abe, A., Innan, H., & Terauchi, R. (2022). Genome Analysis Revives a Forgotten Hybrid Crop Edo-dokoro in the Genus Dioscorea. Plant and Cell Physiology, pcac109. https://doi.org/10.1093/pcp/pcac109
Abstract
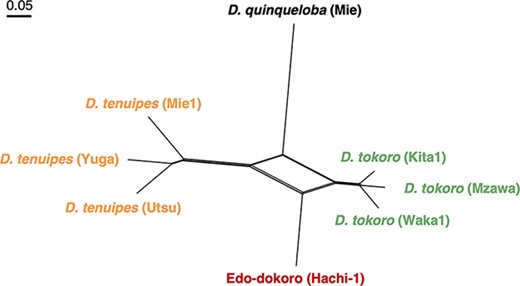
A rhizomatous Dioscorea crop “Edo-dokoro” was described in old records of Japan, but its botanical identify has not been characterized. We found that Edo-dokoro is still produced by four farmers in Tohoku-machi of Aomori Prefecture, Japan. Rhizomes of Edo-dokoro are a delicacy to the local people and are sold in the markets. Morphological characters of Edo-dokoro suggest its hybrid origin between the two species, D. tokoro and D. tenuipes. Genome analysis revealed that Edo-dokoro is likely originated by hybridization of a male D. tokoro to a female D. tenuipes, followed by a backcross with a male plant of D. tokoro. Edo-dokoro is a typical minor crop possibly maintained for more than 300 years but now almost forgotten from the public. We hypothesize that there are many such uncharacterized genetic heritages passed over generations by small scale farmers that await serious scientific investigation for future use and improvement by using modern genomics information.